Buy 5F-CUMYL-PINACA Online
Buy 5F-CUMYL-PINACA Online, The products were analyzed by gas chromatography-mass spectrometry.
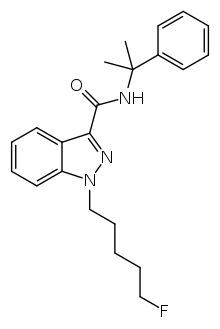
In some of the e-liquids an unknown compound was detected which was identified as the SC 5F-Cumyl-PINACA (1-(5-fluoropentyl)-N-(2-phenylpropan-2-yl)-1H-indazole-3-carboxamide) by nuclear magnetic resonance analysis.
To investigate the phase I metabolism of this new class of compounds, 5F-Cumyl-PINACA and its non-fluorinated analog Cumyl-PINACA were incubated with pooled human liver microsomes (pHLM).
Cumyl-PINACA was additionally ingested orally (0.6 mg) by a volunteer in a controlled self-experiment. To assess the relative potency of Cumyl-PINACA a set of SCs were characterized using a cAMP assay.
Metabolism of 5F-Cumyl-PINACA and Cumyl-PINACA showed similarities with AM-2201 and JWH-018. The main metabolites were formed by hydroxylation at the N-pentyl side chain.
The main metabolites detected in the volunteer’s urine sample were the same as in the pHLM assay. All SCs tested with the cAMP assay were full agonists at the CB1 receptor.
Cumyl-PINACA was the most potent SC among the tested compounds and showed an EC50 value of 0.06 nM.
Buy 5F-CUMYL-PINACA Online, The products were analyzed by gas chromatography-mass spectrometry.
In some of the e-liquids an unknown compound was detected which was identified as the SC 5F-Cumyl-PINACA (1-(5-fluoropentyl)-N-(2-phenylpropan-2-yl)-1H-indazole-3-carboxamide) by nuclear magnetic resonance analysis.
To investigate the phase I metabolism of this new class of compounds, 5F-Cumyl-PINACA and its non-fluorinated analog Cumyl-PINACA were incubated with pooled human liver microsomes (pHLM).
Cumyl-PINACA was additionally ingested orally (0.6 mg) by a volunteer in a controlled self-experiment.
To assess the relative potency of Cumyl-PINACA a set of SCs were characterized using a cAMP assay.
Metabolism of 5F-Cumyl-PINACA and Cumyl-PINACA showed similarities with AM-2201 and JWH-018. The main metabolites were formed by hydroxylation at the N-pentyl side chain.
The main metabolites detected in the volunteer’s urine sample were the same as in the pHLM assay. All SCs tested with the cAMP assay were full agonists at the CB1 receptor.
Cumyl-PINACA was the most potent SC among the tested compounds and showed an EC50 value of 0.06 nM.
Reviews
There are no reviews yet.